| Fungus are eukaryotic organism and they are classified into two main groups that is yeast and molds. Its cell wall is made up of chitin. The fungal structures include mycelium, sporangiospore, spores etc. The Lactophenol Cotton Blue wet mount is simple and widely used staining method for fungi. |
| |
| Lactophenol Cotton Blue Stain (LCB) |
Cotton Blue 0.05g
|
Phenol Crystals 20g
|
Glycerol 40ml
|
Lactic Acid 20ml
|
| Distilled water 20ml |
Method
Preparation of staining requires two days. |
1. Dissolve the Cotton Blue in distilled water and leave overnight to eliminate insoluble dye.
2. Next day, add phenol crystals to the lactic acid in a glass beaker and stir it on magnetic stirrer until the phenol is dissolved.
3. Add the glycerol and filter the cotton blue solution into the Phenol + Glycerol + lactic acid solution.
4. Mix and store at room temperature. |
|
| The main components of LCB staining are : |
1.Phenol: Fungicidal in nature
2.Lactic Acid :Preserves fungal structures
3.Cotton Blue: Stains the chitin in the fungal cell walls & the cytoplasm (in light blue). |
|
| Staining of Clinical Specimens (Non-keratinized) Procedure (LCB Staining) |
1.Place a drop of 70% alcohol on the slide.
2.Add the specimen to the drop of alcohol.
3.Add one or two drops of Lactophenol Cotton Blue Stain before alcohol gets off.
4.Place the coverslip on the drop avoiding air bubbles to be trapped.
5.Examine under Microscope using 10X and 40X objective. |
|
| Staining of fungus from culture |
1.Take a grease free slide.
2.Add a drop of lactophenol cotton blue solution on a slide.
3.Sterilize the inoculation loop or needle and cool it then transfer mycelial growth onto the LCB stain and press it gently so that it easily mix with the stain.
4.Take a clean cover slip and with the help of a forcep place the cover slip on mycelial gowth + LCB.
5.With the help of blotting paper, wipe the excess stain .
6.Observe the preparation under low & high power objectives of the microscope. |
|
| Slide Culture Technique |
| For accurate identification of fungi, it is required that the precise arrangement of the conidiophores and the way in which the spores are produced is essential. The simple method of slide culturing used widely is described here, which permits fungi to be studied virtually in-situ with as little disturbance as possible. |
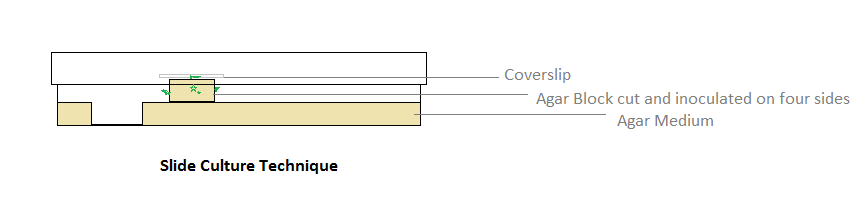 |
| Procedure: |
1.With the help of a sterile blade cut out an agar block (6 x 6 mm) enough to fit under the coverslip.
2.Flip the block up onto the surface of the agar.
3.Inoculate the sides of the agar block with spores or mycelia of the fungus to be grown.
4.Flame the coverslip and place it on agar block.
5.Incubate at 26OC until growth and sporulation take place.
6.After attaining the growth, remove the cover slip from the agar block.
7.Apply a drop of 95% alcohol as a wetting agent and gently lower the coverslip onto a small drop of Lactophenol cotton blue on a grease free glass slide.
8.The slide can be left overnight to dry and later sealed with nail polish.
9.When sealing with nail polish use a coat of clear polish followed by one coat of red coloured polish. |
|
| Exercise |
| Q1. Draw a well labelled diagram of Aspergillus niger and Candida albicans |
| References |
Leck Astrid, 1999. Preparation of Lactophenol Cotton Blue Slide Mounts, Community Eye Health. 1999; 12(30): 24
http://www.generalmicroscience.com/microbial-laboratory-techniques/staining-fungus-using-lactophenol-cotton-blue/
|
|